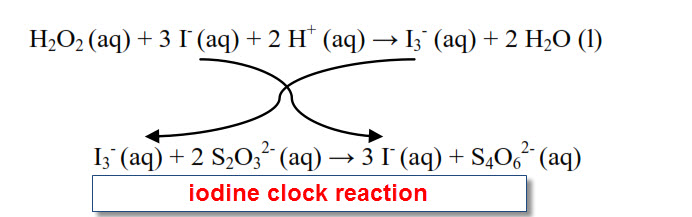
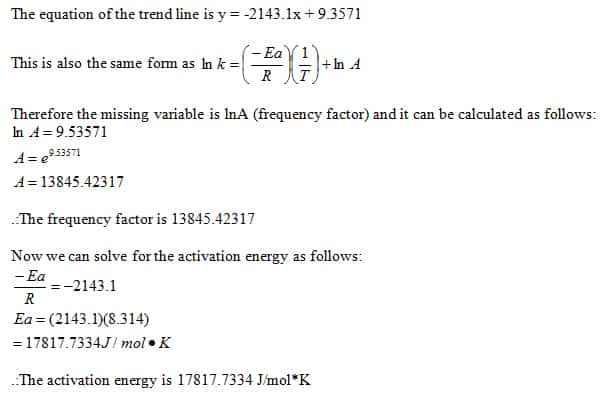Iodine+Clock+Part+A+Sp21 - Iodine Clock Reaction Part A Stephanie R. Geggier New York University, - Studocu

A Closer Examination of the Mechanism of the Hydrogen Peroxide Iodine-Clock Reaction with Respect to the Role of Hypoiodite Species | Journal of Chemical Education
![SOLVED: The rate law for the iodine clock reaction is given by: Rate = k[IO3-] [I-]2[H+]^2. a) This reaction is third order with respect to H+. b) This reaction is first order SOLVED: The rate law for the iodine clock reaction is given by: Rate = k[IO3-] [I-]2[H+]^2. a) This reaction is third order with respect to H+. b) This reaction is first order](https://cdn.numerade.com/ask_previews/494c958b-2794-4a7f-861f-022f5f445939_large.jpg)
SOLVED: The rate law for the iodine clock reaction is given by: Rate = k[IO3-] [I-]2[H+]^2. a) This reaction is third order with respect to H+. b) This reaction is first order

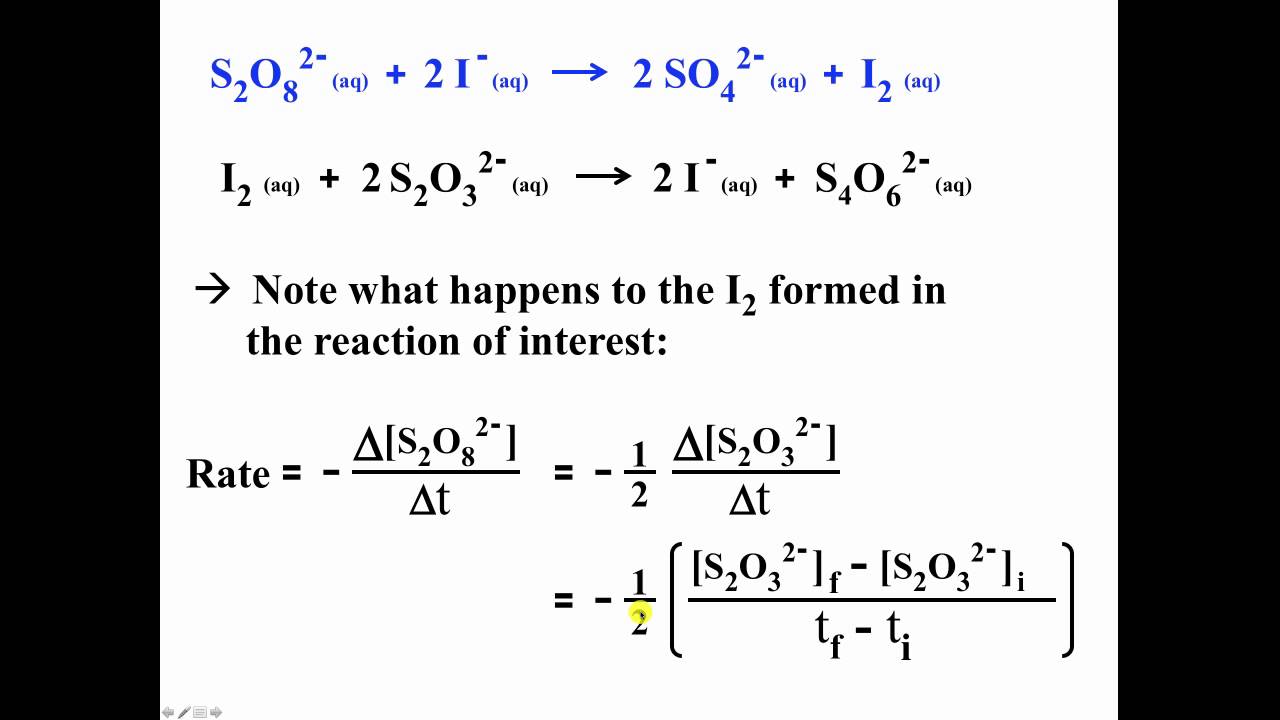
Exp16calcs W - lab report - Chemical Kinetics: The Iodine-Clock Reaction: S 2 O 82 −(aq) + 2 I−(aq) - Studocu

The general reaction scheme for an iodine clock reaction: (red) species... | Download Scientific Diagram

A Microscale Approach to Chemical Kinetics in the General Chemistry Laboratory: The Potassium Iodide Hydrogen Peroxide Iodine-Clock Reaction | Journal of Chemical Education

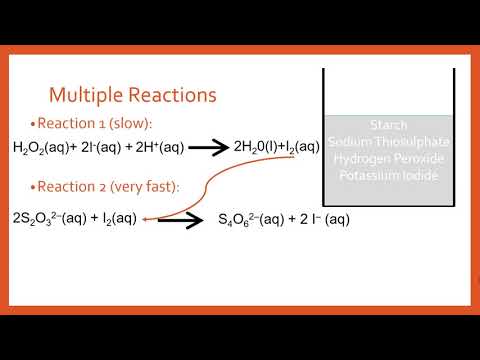
1. Ionic Formula Dissociation Equations. 3I 2 (aq) + 3H 2 O (l) → 6I − (aq) + 3HSO 4 − (aq) + 6H + (aq) fast Demonstration- Iodine clock reaction IO ppt download